
Pyramid logo
Reference: Hellinger, Lisa, https://sites.google.com/site/sed695b4/projects/discrepant-events/fermentation---bewitched-balloons
teacher notes
student documents
career related resources
what materials are needed
- balloons - 1 per flask
- 1000 ml flasks - 2 each
- sugar
- salt
- 400 ml warm water
- dry yeast - 2 packages (record the quantity in grams)
- print this data collection sheet
what are the steps
- Prepare 2 equal size small plastic bags - one with a package of yeast and the other with an equal amount of sugar- label them both with the #1
- Prepare 2 equal size small plastic bags - one with a package of yeast and the other with an equal amount of salt - label them both with the #2
- Do not tell students what the bags contain
- Prepare 200 ml of warm water in each flask
- Ask a volunteer to help and have them pour the contents of both bags labeled one into one flask of warm water. Repeat with the bags labeled 2 in the second flask of warm water
- Students add 50 ml of warm water to each dry sample and stir
- Attach a balloon over the mouth of each flask
- Students can record a time lapse video of the results
break point: 45 minute lesson plan
What if...? Experiment variations
Reference: Great Lakes Bioenergy Research Center - www.glbrc.org/education
Designing individual experiments (pages 10-12) (25-50 minutes).
Students will work to design individual experiments to test methods for improving fermentation of alternative feedstocks. In order to brainstorm experiment ideas, students should work to complete Experimental Pre- Design Questions (page 10) individually, perhaps as homework, or in pairs. The Experimental Design Questions (pages 11-12) should be completed by students in small groups or pairs. Below is a list of possible changes students could make:
- pH
- Length of time the sample runs, measure rates over 24 hrs
- Feedstock choice (fruit juice, flowers, grasses, etc)
- Feedstock concentrations
- Try using a fungus, such as compost fungus, as a biodegrader
- Boil or freeze the sample feedstock before fermenting
- Temperature or concentration of the yeast solution
- Use enzymes (amylase or cellulase for example). See Teacher Tips (pages 7T-8T) for more information. Below are some options:
- Cellulase from Flinn Scientific (www.flinnsci.com). Order #: C0172, $33.95 for 25g at the time of publication in 2010.
- Alpha Amylase from Carolina Biological (www.carolina.com). Order #: 202350, $28.95 for 100g at the time of publication in 2010. Depending upon your students' comfort level designing experiments you may need to help them through the steps of writing a formal procedure, designing a data table or data collection system, recording observations, analyzing and displaying their results, and drawing conclusions.
Questions
1. Yeast cells are alive. What do they “eat" to get the energy they need to grow? What “waste products" do yeast cells produce during fermentation?
2. In this experiment you determined how fast Baker's yeast ferments sugar by measuring how much carbon dioxide was produced. How else could you measure the rate of the fermentation reaction?
3. How long did it take before the yeast started to make carbon dioxide from the sugar (“lag time")? Why do you think the reaction did not start right away?
4. Once fermentation began, the carbon dioxide that was produced pushed most of the air out of the reaction bottle. How could yeast cells live without oxygen?
5. What do you think would happen if you added more or less sugar to the reaction bottle at the start of the experiment? How could you test your hypotheses?
6. Why did you add warm water to the reaction bottle? What do you think would happen if you used cold water? How could you find out what water temperature makes yeast ferment sugar at the fastest rate?
7. What else do you think could make the yeast fermentation reaction go faster? What could slow it down? Think of experiments that could help you find out.
Learn more by doing
Make your own mini fermenter T
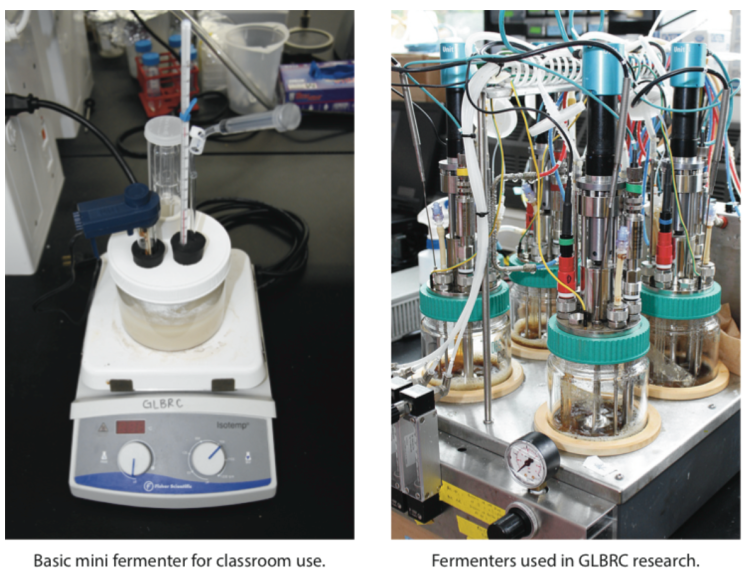
This mini fermenter can be used to conduct small-scale fermentation investigations or demonstrations similar to research done by GLBRC scientists. The design allows for students to use simple techniques and classroom-grade probes to collect data during fermentation on a range of variables, such as ethanol concentration, CO2 production, temperature and pH. The complete minifermenter can be built with readily-available supplies for approximately $20 (detailed supplies list included with instructions).

