There are 2 common definitions of biomaterials:
- A material derived from, or produced by, biological organisms like plants, animals, bacteria, fungi and other life forms. These are also called biologically derived materials.
- A material used for a biological purpose such as a biomedical application like treating an injury or growing biological cells. These may be synthetic.
There are many fascinating and exciting biomaterials! Here are some of the most common and abundant biomaterials produced by living organisms. But wait! These are also a class of materials known as polymers. A polymer is a macromolecule that is composed of monomers that are assembled biologically. It's like making a chain using many links that can be the same or different.
To show it schematically, here is a monomer and a polymer:
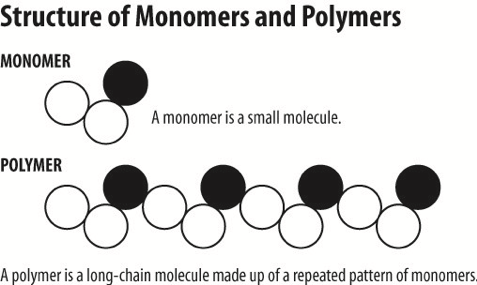
Here are some of the most abundant (and totally awesome) polymeric biomaterials:
Nucleic acids (DNA and RNA)
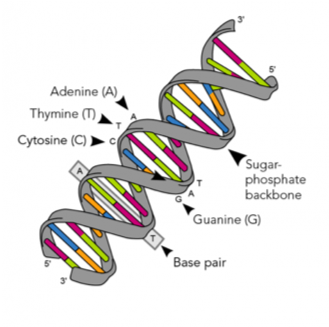
Image Source: What is DNA? | Facts | yourgenome.org
Yes, your DNA and RNA are polymers (of nucleotides) and not only make up your genes and help shape who you are, they are cool biomaterials. Engineers like Biological Engineers have designed DNA to self-assemble into fascinating structures. Here are some 3D DNA nanoscale octahedrons and a DNA smiley face!

Images of actual engineered DNA octahedrons taken using a transmission electron microscope. Each octahedron measures ~20nm in diameter. This means you could fit over a million of these in the diameter of one of your hairs.

Have a nice DNA! A DNA smiley face made of engineered DNA. Image taken transmission electron microscope.
Image Source: Rothemund, P. W. K. (2006). Folding DNA to create nanoscale shapes and patterns. Nature, 440(7082), 297-302. doi:http://dx.doi.org.ezaccess.libraries.psu.edu/10.1038/nature04586
Proteins
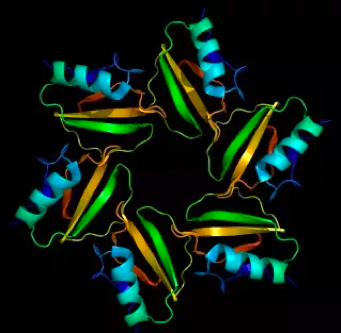
Image Source: http://beautifulproteins.blogspot.com/
Proteins are polymers of amino acids and are made by cells using DNA as the blueprint. Some look like works of art! Like the Proteinase Inhibitor CI-2 from barley seeds shown above. There are several types of proteins:
- Antibodies. Defend the body against intruders like bacteria and viruses.
- Contractile. Responsible for muscle contraction.
- Enzymes/catalysts. Facilitate biochemical reactions like digestion.
- Messenger proteins that coordinate biological processes like insulin production.
- Structural. Create mechanical support for cells and tissue like collagen.
- Storage. Provide amino acids for the body to use later.
- Transport. Carrier proteins that move molecules throughout the body like Hemoglobin that transports oxygen.
Proteins have structure that is important to their function. Proteins found in body tissue or those used in foods provide mechanical properties often by controlling the state of water. Some proteins can bind water so it cannot flow easily - like the water in Jello! Jello, made from the gelatin protein, is full of water, but you cannot squeeze it out!
Protein enzymes can also be complex machines like biomolecular motors. Did you know that your muscles contract because of the activation of billions of biomolecular nanoscale motor proteins that pull muscle fibers together like pulling in a rope?
Here is a cool nanoscale biomolecular motor protein. It is the myosin IV motor proteins that is involved in muscle contraction. (A) is an illustration of the motor protein; (B) is an illustration of a set of proteins (interior) engaging with muscle fiber (exterior); (C) a close up of the motor protein structure showing the 2 motor domains that move to contract muscle.
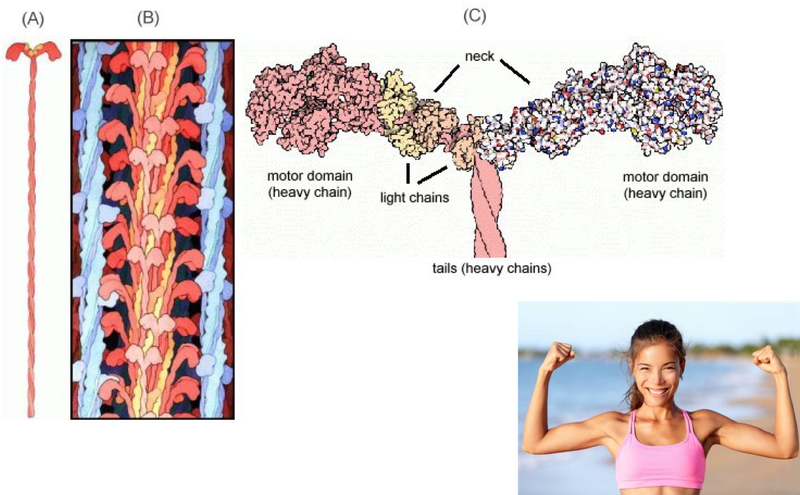
Image Source: animation of F0 rotation (callutheran.edu)
Polysaccharides
Polysaccharides are polymers of sugars. The most common and abundant polysaccharide is cellulose. You may know it as cotton! Cellulose is the major component in plants but also made by bacteria and other organisms. Cellulose is a simple polymer of glucose. Hemicelluloses are also polymers of sugars but include many different sugars like xylose, arabinose, mannose, galactose and rhamnose.

Image Source: Zagis USA to Invest $75 Million in Two New Louisiana Textile Mills (kplctv.com)
Polysaccharides are also found throughout the body and, like proteins (often with them), provide structure and support for cells and tissue to grow. Many polysaccharides are also used in making processed foods like spaghetti sauce and ice cream where they, again, control the state of water. Examples include xanthan gum, gellan gum and carboxymethyl cellulose. Another abundant polysaccharide is starch found in potatoes and corn. Starch is often added to gravy to make it thicker. Are you getting hungry for mashed potatoes and gravy?
Here is cellulose (cotton), a polymer of glucose. The 2-glucose repeat unit of cellulose is called cellobiose. Note how each glucose molecule in the chain is rotated. This gives the molecule some rigidity and a somewhat linear configuration.
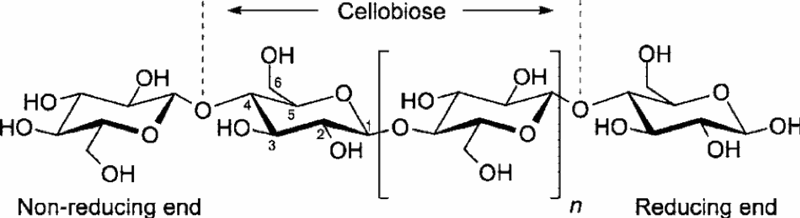
Image Source: Islam, S., Bhuiyan, M. A., R., & Islam, M. N. (2017). Chitin and chitosan: Structure, properties and applications in biomedical engineering. Journal of Polymers and the Environment, 25(3), 854-866.
Polyphenols
Polyphenols are polymers of phenol (see below left, a benzene ring with a hydroxyl group) or phenol-like molecules. Lignin contained in plants is a polyphenolic compound and the second most abundant polymer produced on earth (after cellulose).

One form of the polymer of lignin is shown here.
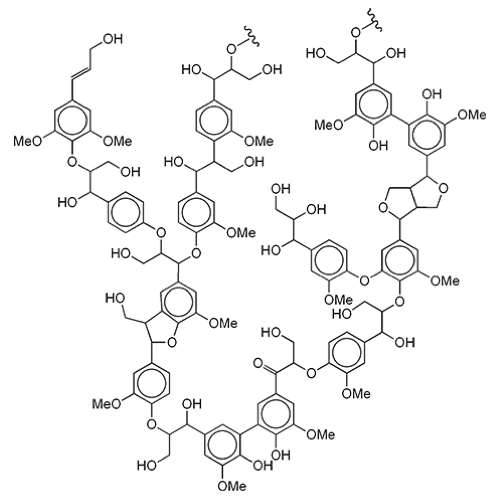
Image Source: J. L. Wertz, M. Deleu, S. Coppée, A. Richel, Hemicelluloses and Lignin in Biorefineries. Taylor & Francis Group, 2018
Polyphenols also include many food substances like flavonoids (found in apples, onions, cabbage, chocolate), phenolic acids (found in many fruits, vegetables, whole grains and seeds), polyphenolic amides (found in chili peppers and oats) and other polyphenols (found in red wine, berries, flax seeds, sesame seeds and whole grains). Such polyphenols are thought to offer numerous health benefits.
Composites
These biologically derived polymers in nature are usually used together by organisms to create composites that have some needed physical, mechanical or chemical property. A composite material is a material made from two or more different materials.
For example, polysaccharides are the most abundant natural polymer on earth as they make up ~55-75% of the weight of plants. These polysaccharides are known as cellulose and hemicelluloses, which make up ~40%-50% and ~15%-25%, respectively, of the plant's dry weight. Woody plants combine them with the polyphenol lignin to make the composite material wood. The content of lignin is ~20%-25%. So when you look at a tree, it is typically over 75% cellulose, hemicellulose and lignin! The assembly is intricate as shown schematically below.

Question: How can plants like trees create such intricate structures ranging from the molecular and nano scales all the way to the macro scale including leaves, bark and trunks? What can we learn from these materials and their assembly to help engineer new sustainable materials that do not pollute our ecosystem, especially at the end of their use?
Fermentation and the production of biomaterials by microorganisms
Fermentation is often referred to as a process of using microorganisms such as bacteria and/or fungi to covert a carbon source such as a sugar into some product like a gas, fuel, polysaccharide, protein or some other molecule.
The process of fermentation typically involves the use of a vessel to hold the liquid medium that provides the carbon and other nutrients to the microorganisms so they can grow and produce the desired product. The vessel can have inputs (like additional carbon, nutrients, air, water, heat or cooling) and outputs (like cells and/or the desired product, which may need to be purified from the cells and medium). See a real fermentation system below located in the Penn State Huck Institutes of the Life Sciences CSL Behring Fermentation user facility.

Fermentation is used to make countless products like foods (yoghurt, wine, beer, cider, buttermilk, sauerkraut, bread, cheese, cocoa, polysaccharide fiber additives and thousands of others), fuel (ethanol) and pharmaceuticals (vaccines, antibiotics, hormones, drug molecules). Bacteria are used to manufacture many polysaccharide fiber food additives like gellan gum and xanthan gum produced by Sphingomonas elodea and Xanthomonas campestris, respectively. Below is a scanning electron microscope image of a single bacteria (Komagataeibacter xylinus) producing polysaccharide fibers (in this case, cellulose).
Why all the excitement over biologically derived materials?
To understand the significance and wonder of natural biologically derived materials, we first must understand a little bit of how life evolved. Single celled organisms appeared about 3.8 billion years ago and evolved into bacteria, archaea, and eukaryota. Eukaryota then evolved into protista (single celled organisms), fungi (single or multicelled organisms) and plants and animals (multicelled organisms). These cells produced polysaccharides, proteins and, in some cases, polyphenolic compounds. So life, for billions of years, has been composed of, and has existed within, these compounds. For example, trees and humans have polysaccharides in their tissues. When these organisms die, other organisms can degrade all of the organic matter produced by these organisms back into carbon, closing the carbon cycle and allowing life to exist sustainability.
Humans, however, are very clever! They have created artificial materials that do not exist in nature that have outstanding properties. Take plastic, for example. Plastic is cheap because of the wide availability of petroleum and can be thermally molded into many shapes. It is insoluble and both water and oil resistant making it a great package. But since it is synthetic, nature cannot easily degrade it, and it results in pollution that endangers our ecosystem and health. Many other human made materials are like this.
If we can make products using biologically derived materials in ways that do not alter their chemical composition, or do so in a way that still allows microorganisms in nature to degrade it, we can improve our environment and human health. Many such products exist, but they can, in some cases, be more expensive or not perform as well as their synthetic counterparts. In some cases, the natural material can perform better. It depends on the application.
Society needs people to develop new materials and processes for making sustainable products! This can be your career! You can be an inventor! You can be a scientist or engineer and help make our civilization sustainable! It is up to you to make a difference!
To do this you will need to learn about science, engineering, mathematics and other disciplines like ethics, sociology, psychology, history, business, politics and other disciplines. You will also need tools. One is life cycle analysis!

Life cycle analysis
Unlike synthetic materials like plastics derived from petroleum and other unsustainable feedstocks, biologically derived materials offer the possibility for ecologically compatible materials, manufacturing and use that is compatible with our ecosystem and human health. Assessing the impact of a product on our environment requires a complex analysis called life cycle analysis where all the materials, energy, infrastructure, labor, etc., are assessed and how those materials and processes consume energy, produce atmospheric carbon and pollute our environment while providing some benefit. Such analyses also attempt to assess the impact on human based climate change.
Imagine a simple product like a stainless steel spoon. What does it take to make it? The metals and other materials (nickel, iron ore, chromium, silicon, molybdenum, and others) need to be mined, and energy (carbon) and machinery are used to process and purify them. The metals need to be melted and combined and formed in a mold requiring more energy (carbon) and machinery. The spoon then must be packaged and transported to the customer requiring more energy (carbon), materials (packaging) and vehicles. The spoon must be designed using computers and many people must operate the numerous machines involved. All these processes result in pollution. All to make a spoon. When the spoon is broken or no longer wanted, it may be recycled (rarely) or discarded wasting all the materials, energy consumed and pollution produced.
Now imagine a wooden spoon. As the tree grows, it consumes carbon (rather than produce it) and provides oxygen. However, the wood may need to be dried consuming energy, and shaped using a machine (also consuming energy). The spoon may also need to be packaged and transported. But wooden spoons can be made locally since trees grow anywhere, reducing transportation. When you are done with the spoon, you can discard it and it will be consumed by nature as a nutrient and not introduce any pollution. But how will the wooden spoon perform relative to the stainless steel one? This kind of analysis is life cycle analysis.
Time to be a scientist!

Image Source: Doutorados em Portugal têm emprego quase garantido - Uniarea
Let's examine a new material for wound care!
1. Polysaccharides for wound healing
People have explored natural materials to aid in wound healing for thousands of years. In ancient Egypt, around 1500 BC, lint in combination with grease and honey were used to treat wounds. The lint was a fiber material like cotton (cellulose polysaccharide), linen (cellulose polysaccharide) and wool fibers (keratin protein) used to absorb wound extrudates and blood. Grease was used as a barrier and honey an antimicrobial agent. Honey is used today with remarkable results (for example, Active Leptospermum Honey is now a common product called Medihoney).
In the 1890's Johnson and Johnson began producing cotton gauze. Many polysaccharide compositions have been examined since for wound care including bacterial cellulose, carboxymethyl cellulose, starch, calcium alginate, chitosan, gellan gum, xanthan gum and blends of these and other materials like proteins (gelatin, collagen, etc.). Natural and some modified polysaccharides tend to exhibit excellent biocompatibility (safe for use on or in humans) since their structure is often similar to polysaccharides found in human tissues.
Currently, researchers are trying to develop new materials that can effectively protect wounds, keep wounds hydrated, absorb or allow the removal of extrudates, promote clotting and wound healing. Polysaccharides are ideal for hydrating wounds as they can bind water and become hydrogels. A hydrogel is a hydrophilic (water binding) polymer that contains a significant amount of water but is generally stable in water, at least for a time. Jello is an excellent example of a hydrogel made from the protein gelatin. Only 0.5-2% (weight/weight in water) of gelatin is required to form a solid gel. Some hydrogels are elastic and can stretch before breaking (like certain kinds of gellan) while some are brittle and tear easily (like gelatin). Elastic gels are desired for wound care as they are more mechanically stable.
2. What is a hydrogel, really?
A hydrogel is a material that can bind water. When water exists in a materials it can be either free (flow easily, like water from a faucet) or bound (the water molecule is attached to another larger molecule, like the water in Jello that cannot be squeezed out as it could from a wash cloth). To make a stable hydrogel, you need enough material (like a soluble polysaccharide) to begin to bind the water and to allow for some binding between polysaccharides. This is shown below.
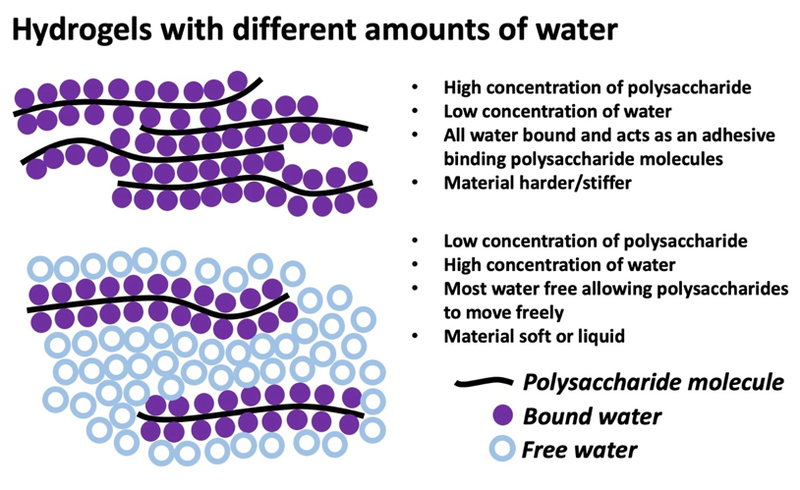
3. What do we want to learn?
Recent research has shown a novel polysaccharide gellan gum to be useful for wound care treatment []. At a minimum, wound care materials need to be mechanically stable in order to protect the wound and hydrate the wound site. In this exercise you will learn how to make a gellan hydrogel and will test its mechanical properties. Specifically, you will do a simple shear test to see how hydrogel density impacts the gellan's shear strength.
4. What you will need
You will need the following materials and instruments to do this experiment:
- 500mL water
- Wood or silicone stir
- Scale
- Rice, oats or something used to apply weight
- 5 teaspoons Gellan gum, ONLY High Acyl, LT-100 (available from the Modernist Pantry and other sources)

- Teaspoon

- Stove or hot plate to boil water

- High speed blender

- 1L shallow metal pan

- Silicone Butter Mold 4 Cavities Rectangle Large Collins Ice Cube Trays (5.25x1.25x1.25 inches cubes)

- 2 thick nails (16d penny common nails work best) or thick toothpicks.

- Wire

- Plastic dish or cup

- Lab support base and clamp

- Ruler

- Timer

Process for making your hydrogels
You will end up with a material that looks like this when you remove it from the mold (shown on a wooden cutting board):

Setup for testing your hydrogels
In this experiment we will measure the shear strength of your gellan hydrogel material. Your final setup should look like the figure below. The nails and loops holding them should be level as should the cup. The cup will hold the rice or other material you use to test how much weight your hydrogel can hold. You should bend the wire so that the loops are positioned close to the hydrogel and so that the system is stable under the applied load.

(All images for this experiment are sourced from Amazon.com)
Process for testing your hydrogels
Step 1: To test the gellan hydrogen shear strength as a function of density, you will first calculate the density (D) in (g/mm3). Using the scale, weigh each sample to determine the weight (W) in grams (g). Then carefully measure the length (l), width (w) and thickness (t) of each sample in millimeters (mm). To do this accurately, measure each side 3 times and take the average. Density is then given by: D = W/l´w´t. Record the values on the worksheet. If you know the dimensions of the mold, you can use them for l and w, but you must measure t.
Step 2: Weigh the empty cup and wire fixture used in the experiment. Record the value on the worksheet.
Step 3: Measure the diameter of the nail (DN) or other object used to support the sample in mm. Record the value on the worksheet.
Step 4: Insert the nails into the sample as shown above ~ 1 inch from the ends making sure they are parallel to the edge and place in the fixture as shown. The nail should penetrate through the thickness (t) of the sample. Specifically, the nail should be penetrating through the side of the sample whose thickness was measured to be t. This is the poured thickness of the sample in the mold after cooling.
Step 5: Assemble the rest of the fixture as shown.
Step 6: Pour 1/8 cup of rice into the cup and wait 20 seconds to see if the sample can support the weight. If it can, add another 1/8 cup of rice and wait another 20 seconds. Continue until the sample breaks. When the sample breaks, remove the last 1/8 cup of rice.
Step 7: Weigh the cup filled with rice and subtract the weight of the empty cup and wire. This is the maximum supported weight (Wmax). Record the values on the worksheet.
Step 8: Calculate the maximum shear stress Smax = Wmax/DN´t (g/mm2). To convert this value to Pascals (Pa) multiply by 9806. Here is an example:
A 16d penny common nail is 4.1mm (DN=4.1mm). If your sample was 15mm thick, (t=15mm). If your sample could support 100g of rice thant would be 100 g of force (Wmax=100g). Thus Smax=100g/(4.1mm)(15mm) = 1.63g/mm2 or Smax (Pa) = 1.63*9806 Pa or 15,983 Pa of 15.98kPa. This is a common number for a maximum shear stress (which is shear strength) for a gellan material.
Step 9: Do this measurement and calculation for all samples and complete the worksheet.
Step 10: Analyze the data. Did you see a trend with density? If not what could be the cause?
Worksheets (for print out).
Complete the worksheet. Be prepared to discuss.
Competition! Who made the strongest hydrogel? Who made the weakest? What was the difference in the processing or testing? Analyze!
PDF document, 663.8 KB
PDF document, 663.8 KB
Word 2007 document, 6.6 MB
PDF document, 693.1 KB
PDF document, 702.1 KB
Word 2007 document, 6.6 MB
Word 2007 document, 6.6 MB
PDF document, 702.1 KB

 word format
word format